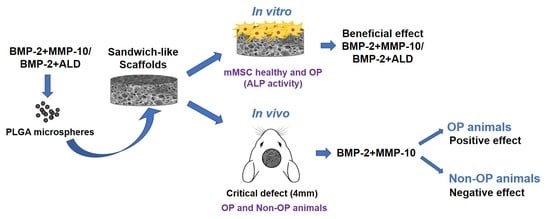
The Bone Regeneration Capacity of BMP-2 + MMP-10 Loaded Scaffolds Depends on the Tissue Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microspheres Preparation and Characterization
2.1.1. Microsphere Preparation
2.1.2. Microspheres Loading Efficiency
2.2. Preparation and Characterization of Electrospun Meshes
2.2.1. PLGA Functionalization with ALENDRONATE
2.2.2. Electrospun Meshes Preparation
2.2.3. ALD-Loaded and Blank Meshes Characterization
2.3. Foams Preparation and Characterization
2.3.1. Foam Preparation
2.3.2. Foams Wettability and Degradation
2.3.3. In Vitro Drug Release
2.4. Osteoporosis Animal Model
2.5. Cell Isolation and Characterization
2.5.1. Osteoporotic and Normal mMSCs Isolation
2.5.2. Characterization of Isolated Cells
2.6. In Vitro Biological Performance of Chitosan Foams and Electrospun Meshes
2.6.1. Evaluation of Developed Foams Osteogenic Capacity
2.6.2. Evaluation of Cell Viability and Adhesion to Electrospun Meshes
2.7. In Vivo Experiments
2.7.1. Surgical Procedure
2.7.2. In Vivo Drug Release
2.7.3. Histological and Histomorphometric Evaluation
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Performance of Developed Systems
3.1.1. Polymeric Microspheres
3.1.2. Electrospun Meshes
3.1.3. Microsphere-Loaded Chitosan Foams
3.2. Osteoporosis Instauration
3.3. Characterization of OP-Like mMSC and “Healthy” mMSC
3.4. In Vitro Performance of Chitosan Foams and Electrospun Meshes
3.5. In Vivo Evaluation of Sandwich-Like Scaffolds
3.5.1. In Vivo Protein Drug Release
3.5.2. In Vivo Bone Formation Induced by Sandwich-Like Scaffold Containing BMP-2 Alone or Combined with ALD or MMP-10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Michalski, M.N.; McCauley, L.K. Macrophages and skeletal health. Pharmacol. Ther. 2017, 174, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Russow, G.; Jahn, D.; Appelt, J.; Märdian, S.; Tsitsilonis, S.; Keller, J. Anabolic Therapies in Osteoporosis and Bone Regeneration. Int. J. Mol. Sci. 2018, 20, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; López-Noriega, A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J. Control. Release 2015, 207, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lyritis, G.P.; Georgoulas, T.; Zafeiris, C.P. Bone anabolic versus bone anticatabolic treatment of postmenopausal osteoporosis. Ann. N. Y. Acad. Sci. 2010, 1205, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Segredo-Morales, E.; García-García, P.; Reyes, R.; Pérez-Herrero, E.; Delgado, A.; Évora, C. Bone regeneration in osteoporosis by delivery BMP-2 and PRGF from tetronic–alginate composite thermogel. Int. J. Pharm. 2018, 543, 160–168. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; Reyes, R.; Arnau, M.R.; Delgado, A.; Évora, C. In situ gel-forming system for dual BMP-2 and 17β-estradiol controlled release for bone regeneration in osteoporotic rats. Drug Deliv. Transl. Res. 2018, 8, 1103–1113. [Google Scholar] [CrossRef]
- García, P.; Reyes, R.; Segredo-Morales, E.; Pérez-Herrero, E.; Delgado, A.; Évora, C. PLGA-BMP-2 and PLA-17β-Estradiol Microspheres Reinforcing a Composite Hydrogel for Bone Regeneration in Osteoporosis. Pharmaceutics 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Hur, W.; Park, M.; Lee, J.Y.; Kim, M.H.; Lee, S.H.; Park, C.G.; Kim, S.N.; Min, H.S.; Min, H.J.; Chai, J.H.; et al. Bioabsorbable bone plates enabled with local, sustained delivery of alendronate for bone regeneration. J. Control. Release Off. J. Control. Release Soc. 2016, 222, 97–106. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Kim, H.J.; Park, K.; Song, H.R. 3D printed alendronate-releasing poly (caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed. Mater. 2016, 11, 055005. [Google Scholar] [CrossRef]
- Mardas, N.; Busetti, J.; de Figueiredo, J.A.; Mezzomo, L.A.; Scarparo, R.K.; Donos, N. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin. Oral Implant. Res. 2017, 28, 362–371. [Google Scholar] [CrossRef]
- van Houdt, C.I.A.; Gabbai-Armelin, P.R.; Lopez-Perez, P.M.; Ulrich, D.J.O.; Jansen, J.A.; Renno, A.C.M.; van den Beucken, J. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, D.; Weng, W.; Huang, Q.; Zhang, X.; Wen, J.; Wu, J.; Jiang, X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. A comparative evaluation of the systemic and local alendronate treatment in synthetic bone graft: A histologic and histomorphometric study in a rat calvarial defect model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S146–S152. [Google Scholar] [CrossRef]
- Huntley, R.; Jensen, E.; Gopalakrishnan, R.; Mansky, K.C. Bone morphogenetic proteins: Their role in regulating osteoclast differentiation. Bone Rep. 2019, 10, 100207. [Google Scholar] [CrossRef] [PubMed]
- Little, D.G.; McDonald, M.; Bransford, R.; Godfrey, C.B.; Amanat, N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2005, 20, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.B.; Larsson, D.; Mrkonjic, F.; Isaksson, H.; Kumar, A.; Lidgren, L.; Tägil, M. Gelatin-hydroxyapatite-calcium sulphate based biomaterial for long term sustained delivery of bone morphogenic protein-2 and zoledronic acid for increased bone formation: In-Vitro and In-Vivo carrier properties. J. Control. Release Off. J. Control. Release Soc. 2018, 272, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Baek, H.R.; Lee, K.M.; Zheng, G.B.; Shin, S.J.; Jin, Y.Z. The inhibitory effect of zoledronate on early-stage osteoinduction by recombinant human bone morphogenetic protein 2 in an osteoporosis model. Growth Factors 2015, 33, 220–228. [Google Scholar] [CrossRef]
- Mathavan, N.; Tägil, M.; Isaksson, H. Do osteoporotic fractures constitute a greater recalcitrant challenge for skeletal regeneration? Investigating the efficacy of BMP-7 and zoledronate treatment of diaphyseal fractures in an open fracture osteoporotic rat model. Osteoporos Int. 2017, 28, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef] [Green Version]
- Kosaki, N.; Takaishi, H.; Kamekura, S.; Kimura, T.; Okada, Y.; Minqi, L.; Amizuka, N.; Chung, U.-I.; Nakamura, K.; Kawaguchi, H.; et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem. Biophys. Res. Commun. 2007, 354, 846–851. [Google Scholar] [CrossRef]
- Stickens, D.; Behonick, D.J.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.J.; Schorpp-Kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883. [Google Scholar] [CrossRef] [Green Version]
- Lieu, S.; Hansen, E.; Dedini, R.; Behonick, D.; Werb, Z.; Miclau, T.; Marcucio, R.; Colnot, C. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis. Model. Mech. 2011, 4, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Bord, S.; Horner, A.; Hembry, R.M.; Compston, J.E. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: Potential roles in skeletal development. Bone 1998, 23, 7–12. [Google Scholar] [CrossRef]
- Mao, L.; Yano, M.; Kawao, N.; Tamura, Y.; Okada, K.; Kaji, H. Role of matrix metalloproteinase-10 in the BMP-2 inducing osteoblastic differentiation. Endocr. J. 2013, 60, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Matilla, L.; Roncal, C.; Ibarrola, J.; Arrieta, V.; Garciá-Penã, A.; Fernández-Celis, A.; Navarro, A.; Álvarez, V.; Gainza, A.; Orbe, J.; et al. A Role for MMP-10 (Matrix Metalloproteinase-10) in Calcific Aortic Valve Stenosis. Arterioscler. Thromb. Vasc. Biol. 2020, 4, 1370–1382. [Google Scholar] [CrossRef]
- Reyes, R.; Rodríguez, J.A.; Orbe, J.; Arnau, M.R.; Évora, C.; Delgado, A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv. 2018, 25, 750–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orbe, J.; Barrenetxe, J.; Rodriguez, J.A.; Vivien, D.; Orset, C.; Parks, W.C.; Birkland, T.P.; Serrano, R.; Purroy, A.; Martinez de Lizarrondo, S.; et al. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation 2011, 124, 2909–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraker, P.J.; Speck, J.C., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Del Rosario, C.; Rodríguez-Évora, M.; Reyes, R.; Simões, S.; Concheiro, A.; Évora, C.; Alvarez-Lorenzo, C.; Delgado, A. Bone critical defect repair with poloxamine-cyclodextrin supramolecular gels. Int. J. Pharm. 2015, 495, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, E.; Ekinci, M.; Ozgenc, E.; Ozdemir, D.I.; Asikoglu, M. Development and Evaluation of Liquid and Solid Lipid Based Drug Delivery Systems Containing Technetium-99m-Radiolabeled Alendronate Sodium. Curr. Radiopharm. 2018, 11, 100–108. [Google Scholar] [CrossRef] [PubMed]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Evora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255. [Google Scholar] [CrossRef]
- Hernández, A.; Sánchez, E.; Soriano, I.; Reyes, R.; Delgado, A.; Évora, C. Material-related effects of BMP-2 delivery systems on bone regeneration. Acta Biomater. 2012, 8, 781–791. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Rodríguez-Évora, M.; Delgado, A.; Reyes, R.; Hernández-Daranas, A.; Soriano, I.; San Román, J.; Évora, C. Osteogenic effect of local, long versus short term BMP-2 delivery from a novel SPU–PLGA–βTCP concentric system in a critical size defect in rats. Eur. J. Pharm. Sci. 2013, 49, 873–884. [Google Scholar] [CrossRef]
- Del Rosario, C.; Rodríguez-Évora, M.; Reyes, R.; Delgado, A.; Évora, C. BMP-2, PDGF-BB, and bone marrow mesenchymal cells in a macroporous β-TCP scaffold for critical-size bone defect repair in rats. Biomed. Mater. 2015, 10, 045008. [Google Scholar] [CrossRef]
- De la Riva, B.; Sánchez, E.; Hernández, A.; Reyes, R.; Tamimi, F.; López-Cabarcos, E.; Delgado, A.; Evora, C. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J. Control. Release Off. J. Control. Release 2010, 143, 45–52. [Google Scholar] [CrossRef]
- Rodríguez-Évora, M.; García-Pizarro, E.; del Rosario, C.; Pérez-López, J.; Reyes, R.; Delgado, A.; Rodríguez-Rey, J.C.; Évora, C. Smurf1 knocked-down, mesenchymal stem cells and BMP-2 in an electrospun system for bone regeneration. Biomacromolecules 2014, 15, 1311–1322. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Faibish, D.; Ott, S.M.; Boskey, A.L. Mineral changes in osteoporosis: A review. Clin. Orthop. Relat. Res. 2006, 443, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deca, A.G.; Belu, I.; Croitoru, O.; Bubulică, M.V.; Manda, C.V.; Neamtu, J. Formulation and In Vitro Evaluation of Alendronate Sodium/PLGA Microspheres for Applications in Bone Related Disorders. Curr. health Sci. J. 2015, 41, 246–250. [Google Scholar] [CrossRef]
- Nafea, E.H.; El-Massik, M.A.; El-Khordagui, L.K.; Marei, M.k.; Khalafallah, N.M. Alendronate PLGA microspheres with high loading efficiency for dental applications. J. Microencapsul. 2007, 24, 525–538. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Sukul, M.; Sahariah, P.; Lauzon, H.L.; Borges, J.; Másson, M.; Mano, J.F.; Haugen, H.J.; Reseland, J.E. In vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight. Carbohydr. Polym. 2021, 254, 117434. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.J.; Lee, J.Y.; Park, Y.J.; Lee, Y.M.; Young, K.; Rhyu, I.C.; Lee, S.J.; Han, S.B.; Chung, C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Ikono, R.; Li, N.; Pratama, N.H.; Vibriani, A.; Yuniarni, D.R.; Luthfansyah, M.; Bachtiar, B.M.; Bachtiar, E.W.; Mulia, K.; Nasikin, M.; et al. Enhanced bone regeneration capability of chitosan sponge coated with TiO2 nanoparticles. Biotechnol. Rep. 2019, 24, e00350. [Google Scholar] [CrossRef] [PubMed]
- Paspaliaris, V.; Kolios, G. Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019, 2019, 1730978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonyadi, M.; Waldman, S.D.; Liu, D.; Aubin, J.E.; Grynpas, M.D.; Stanford, W.L. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 5840–5845. [Google Scholar] [CrossRef] [Green Version]
- Hidestrand, M.; Richards-Malcolm, S.; Gurley, C.M.; Nolen, G.; Grimes, B.; Waterstrat, A.; Zant, G.V.; Peterson, C.A. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 566–579. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.; Khan, T.S.; Owen, C.; Ciliberti, N.; Grynpas, M.D.; Stanford, W.L. Longitudinal analysis of mesenchymal progenitors and bone quality in the stem cell antigen-1-null osteoporotic mouse. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2007, 22, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Agata, H.; Sumita, Y.; Hidaka, T.; Iwatake, M.; Kagami, H.; Asahina, I. Intra-Bone Marrow Administration of Mesenchymal Stem/Stromal Cells Is a Promising Approach for Treating Osteoporosis. Stem Cells Int. 2019, 2019, 4214281. [Google Scholar] [CrossRef] [Green Version]
- Hayer, S.; Steiner, G.; Görtz, B.; Reiter, E.; Tohidast-Akrad, M.; Amling, M.; Hoffmann, O.; Redlich, K.; Zwerina, J.; Skriner, K.; et al. CD44 is a determinant of inflammatory bone loss. J. Exp. Med. 2005, 201, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; Coathup, M.; Samazideh, S.; Kalia, P.; Silvio, L.D.; Idowu, B.; Blunn, G. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4. Bone Jt. Res. 2017, 6, 358–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, J.-C.; Zang, H.-Y.; Guo, L.-X.; Xue, H.-B.; Liu, X.-D.; Bai, Y.-B.; Ma, Y.-Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept. Signal. Transduct. 2015, 35, 640–645. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-García, P.; Reyes, R.; Pérez-Herrero, E.; Arnau, M.R.; Évora, C.; Delgado, A. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater. Sci. Eng. 2020, 115, 111009. [Google Scholar] [CrossRef] [PubMed]









| Microspheres Type | BMP-2 (µg) | ALD (µg) | MMP-10 (µg) |
|---|---|---|---|
| Blank PLGA | - | - | - |
| PLGA-BMP-2 | 35 µg | - | - |
| PLGA/Chitosan-BMP-2 + ALD | 35 µg | 75 µg | - |
| PLGA-BMP-2 + Low MMP-10 | 35 µg | - | 2.1 µg |
| PLGA-BMP-2 + High MMP-10 | 35 µg | - | 8.5 µg |
| Nomenclature | Loaded Molecules (Dose) | Used Microspheres |
|---|---|---|
| Control foam | - | Blank PLGA |
| BMP-2 foam | BMP-2 (600 ng) | PLGA-BMP-2 |
| BMP-2 + ALD foam | BMP-2 (600 ng) + ALD (75 µg) | PLGA/Chitosan-BMP-2 + ALD |
| BMP-2 + Low MMP-10 foam | BMP-2 (600 ng) + MMP-10 (30 ng) | PLGA-BMP-2 + Low MMP-10 |
| BMP-2 + High MMP-10 foam | BMP-2 (600 ng) + MMP-10 (120 ng) | PLGA-BMP-2 + High MMP-10 |
| Sandwich-Like Scaffolds | ||
|---|---|---|
| Treatment Group | Chitosan Foam  | Electrospun Mesh  |
| BMP | BMP-2 foam | Blank mesh |
| BMP + ALD | BMP-2 + ALD foam | ALD-mesh |
| BMP + MMP-L | BMP-2 + Low MMP-10 foam | Blank mesh |
| BMP + MMP-H | BMP-2 + High MMP-10 foam | Blank mesh |
 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garcia, P.; Reyes, R.; Rodriguez, J.A.; Martín, T.; Evora, C.; Díaz-Rodríguez, P.; Delgado, A. The Bone Regeneration Capacity of BMP-2 + MMP-10 Loaded Scaffolds Depends on the Tissue Status. Pharmaceutics 2021, 13, 979. https://doi.org/10.3390/pharmaceutics13070979
Garcia-Garcia P, Reyes R, Rodriguez JA, Martín T, Evora C, Díaz-Rodríguez P, Delgado A. The Bone Regeneration Capacity of BMP-2 + MMP-10 Loaded Scaffolds Depends on the Tissue Status. Pharmaceutics. 2021; 13(7):979. https://doi.org/10.3390/pharmaceutics13070979
Chicago/Turabian StyleGarcia-Garcia, Patricia, Ricardo Reyes, José Antonio Rodriguez, Tomas Martín, Carmen Evora, Patricia Díaz-Rodríguez, and Araceli Delgado. 2021. "The Bone Regeneration Capacity of BMP-2 + MMP-10 Loaded Scaffolds Depends on the Tissue Status" Pharmaceutics 13, no. 7: 979. https://doi.org/10.3390/pharmaceutics13070979